Herceptin (6-8 mg/kg) : >500mg/dose
Opdivo (PD-1) : >500mg/dose
Avastin (10 mg/kg) : 750 mg/dose
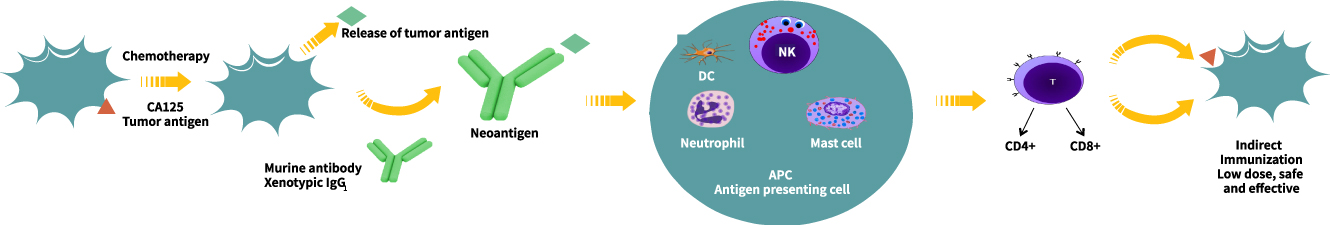
● The neoantigen gets absorbed by dendritic cells, leading to the CA125-specific immune
response from cytotoxic CD8+ T-cells
Oregovomab : 2 mg/dose
AR20.5 (MUC1) : 2 mg/dose
In Phase II study, PFS was prolonged to 41.8 months (p=0.0027) from 12.2 months with just chemotherapy, thus showed potential as a much needed novel first-line treatment
Granted an Orphan Drug Designation by the U.S. FDA
August 2020
602 patients worldwide
Global progress: Dosed the first patient at a trial center in the U.S. in Q4 2020. To date, the trial has been initiated at 130 centers across the globe and enrolled over 330 patients.

The 8th most common cause of death to women1
The status quo of ovarian cancer in China
1. World Cancer Research Fund International. Ovarian Cancer Statistics. September 2022.
2. WHO Globocan
3. Chinese Anti-Cancer Association Gynecological Oncology Committee. Guidelines for the Diagnosis and Treatment of Ovarian Malignant Tumors (2021 edition) [J]. Chinese Journal of Cancer, 2021, Vol. 31, No. 6.